The key and very detailed guide to the harmonized product legislations is the Blue Guide 2022. The information on this page follows the content of the Blue Guide 2022 (click to download).
The history of the EU product legislation
The history of the EU product legislation follows 5 key phases:
1. Old Approach
Detailed legal text with all technical and administrative obligations
2. New Approach (1985)
Essential requirements in the legal text, and technical details in standards (the birth of standardization)
3. Conformity assessment
Required by the various harmonization acts
4. New Legislative Framework (2008)
All elements for conformity assessment (incl. accreditation and market surveillance) are developed
5. New Regulations (2019)
on (i) market surveillance and (ii) mutual recognition of goods
The New Approach and the New Legislative Framework
The New Approach and the New Legislative Framework:
- harmonization of EU rules
- technical specifications must be laid down in harmonized standards – they are voluntary applications, possible technical solutions to the legally binding essential requirements
- products produced in compliance with the harmonized standards will benefit from the presumption of conformity
A harmonized standard is a European standard developed by a recognized European Standards Organization: CEN, CENELEC, or ETSI. It is created following a request from the European Commission to one of these organizations. Manufacturers, other economic operators, or conformity assessment bodies can use harmonized standards to demonstrate that products, services, or processes comply with relevant EU legislation.
If manufacturers choose not to apply harmonized standards, they have the obligation to demonstrate that their products are in conformity with essential requirements by the use of other means of their own choice that provide for the level of safety or protection of other interests required by the applicable legislation. These can be other standards such as national standards, international standards, European standards etc. In these cases the manufacturers do not benefit from the presumption of conformity, but have to demonstrate the conformity themselves. This implies that they demonstrate, in the technical file of a relevant product, in a more detailed manner how the standards or technical specifications they use provide conformity with the essential requirements, for instance by carrying out a more in-depth risk assessment on the product, a gap analysis, etc.
The New Legislative Framework:
- essential or other legal requirements;
- product standards;
- standards and rules for the competence of conformity assessment bodies as well as for accreditation;
- standards for quality management;
- conformity assessment procedures;
- CE marking, accreditation policy; and
- market surveillance policy including the control of products from third countries.
Applicable legislations:
Regulation (EU) 1025/2012 on standardization
Regulation (EC) 765/2008 on accreditation and CE marking
Regulation (EU) 2019/1020 on market surveillance and compliance of products
Mutual Recognition of Goods
Products lawfully marketed in one EU Member State should in principle move freely throughout the EU. In the absence of EU harmonization legislation, EU Member States are free to legislate on their territory subject to the EU Treaty rules on free movement of goods (Arts. 34 – 36).
Barriers to free movement of goods which result from differences in national legislation may only be accepted if
- the national rule of the EU Member State of destination pursues a legitimate public interest objective; and
- the measure restricting or denying access is proportionate, meaning that the measure is appropriate for securing the attainment of the objective and necessary (it does not go beyond what is necessary for attaining the objective).
Applicable legislation:
Regulation (EU) 2019/515 on mutual recognition of goods
The General Product Safety Directive (GPSD)
The GPSD is intended to ensure product safety throughout the EU for all non-food consumer products to the extent that they are not covered by sector-specific EU harmonization legislation. The GPSD has set up the EU Rapid Alert System which is used to quickly exchange information between Member States and the Commission on measures taken against dangerous non-food products (RAPEX).
On 30 June 2021, the Commission adopted a proposal for a new General Product Safety Regulation, to replace the GPSD. (Click here for the latest news on this activity.)
Applicable legislation:
Directive 2001/95/EC of general safety of products
The new market surveillance regulation
Regulation (EU) 2019/1020 on market surveillance and compliance of products entered into force on 16 July, 2021 (MS regulation).
The MS regulation provides:
- clear and uniform rules applying to non-food products and economic operators;
- requirements (infrastructure, organization, legal powers, etc.) to ensure that market surveillance can cope with enforcing EU legislation;
- streamlined market surveillance procedures for controlling products within the EU and at its borders (import controls);
- tools to coordinate activities carried out by national surveillance bodies across the EU (e.g. discussion forums, IT databases, and common market surveillance campaigns).
The MS regulation is a very important piece of legislation, because:
- it identifies the various market actors and their obligations (i.e. manufacturer, distributor, authorized representative, importer etc.); and
- its Annex I. lists all the specific EU harmonization legislations (i.e. WEEE, F-gas, energy label, ecodesign etc.).
The Blue Guide 2022 provides detailed explanation to the terms and definition of the MS regulation.
The EU harmonization legislation applies:
- when the product is placed on the EU market and to any subsequent operation which constitutes making available until it reaches the end-user.
- to all forms of selling. A product offered in a catalogue or by means of electronic commerce has to comply with EU harmonization legislation when the catalogue or website directs its offer to the EU market and includes an ordering and shipping system.
- to newly manufactured products but also to used and second-hand products imported from a third country when they enter the EU market for the first time
- to finished products as defined by the scope of each legislation.
(A product which has been subject to important changes or overhauls aiming to modify its original performance, purpose or type may be considered as a new product. The person who carries out the changes becomes then the manufacturer with the corresponding obligations.)
The territorial scope of the harmonized legislations:
- EU harmonization legislation applies to the Member States of the EU and to certain European territories to the extent necessary to give effect to the arrangements set out in the Accession Treaty of the relevant Member States.
- The Agreement on the European Economic Area is established between the European Union and Iceland, Liechtenstein and Norway. The Agreement extends the internal market to these three EFTA States – commonly known as EEA EFTA States.
- The Customs Union Agreement between the EU and Turkey aims to ensure the free movement of products between the EU and Turkey, by eliminating import controls at the EU-Turkey border on such products.
- The Protocol on Ireland and Northern Ireland of the Agreement on the Withdrawal of the UK from the EU extends also the application of certain Union product legislation to Northern Ireland.Termékkövetelmények a harmonizációs szabályok értelmében
Product requirements under the harmonization legislations
The traceability requirements
The traceability requirements allow tracing the history of the product and support market surveillance. It allows market surveillance authorities to find the liable economic operators and obtain evidence of the product compliance. They include labelling the product and identifying the economic operators in the distribution chain.
The technical documentation and the Declaration of Conformity
The manufacturer must draw up a technical documentation. The technical documentation is intended to provide information on the design, manufacture and operation of the product.
The contents of the technical documentation are laid down, in each Union harmonization act, in accordance with the products concerned.
The technical documentation must be written in a language accepted by the notified body.
The manufacturer or the authorized representative established within the EU must draw up and sign a Declaration of Conformity as part of the conformity assessment procedure provided for in the EU harmonization legislation.
The Declaration of Conformity must contain all relevant information to identify the EU harmonization legislation according to which it is issued, as well as the manufacturer, the authorized representative, the notified body if applicable, the product, and where appropriate a reference to harmonized standards or other technical specifications.
A single declaration of conformity is required whenever a product is covered by several pieces of EU harmonization legislation requiring a Declaration of Conformity.
The single declaration of conformity can be made up of a dossier containing all relevant individual declarations of conformity.
The Declaration of Conformity must be written in a language required by the EU Member States.
The technical documentation and the Declaration of Conformity must be kept for 10 years from the date of placing the product on the market, unless the applicable Union harmonization legislation expressly provides for any other duration.
The CE marking
The CE marking indicates the conformity of the product with the Union legislation applying to the product and providing for CE marking.
The CE marking is affixed on products that will be placed on the EEA and Turkish market, whether they are manufactured in the EEA, in Turkey or in another country.
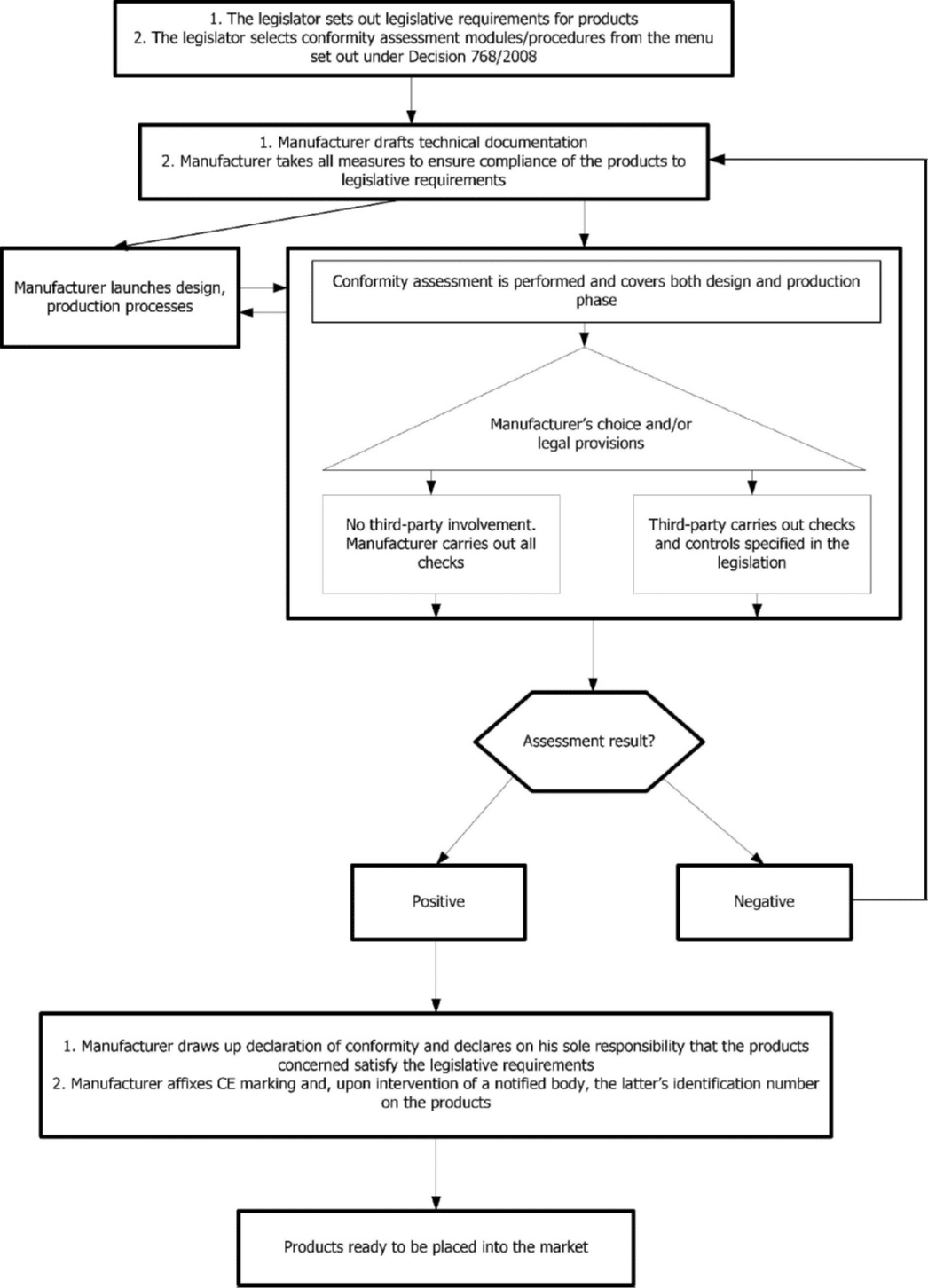
The conformity assessment
The following chart of the Blue Guide 2022 shows the steps of the conformity assessment:
Applicable legislations:
Regulation (EC) 765/2008 on accreditation and CE marking
Regulation (EU) 2019/1020 on market surveillance and compliance of products